Technology capable of sampling water systems to find indicators of fecal matter contamination that are thousandths and even millionths of times smaller than those found by conventional methods is being developed by a team of researchers at Texas A&M University.
Working with a team of collaborators, Vladislav Yakovlev, professor in the Department of Biomedical Engineering, has developed an ultra sensitive detection method that can detect molecules associated with human and animal fecal matter in water systems. These extremely small indicators, he explains, have been traditionally difficult to detect but can signal greater levels of contamination, which can lead to illness and even death.

Professor Vladislav Yakovlev
Read more about Vladislav Yakovlev
The team’s research is funded by the National Science Foundation and is featured in the journal “Proceedings of the National Academy of Sciences.” It details the development of technology that Yakovlev characterizes as affordable, highly sensitive, easy to implement and capable of delivering analysis of water samples in real time. That combination of benefits, he says, gives the system a leg up on other detection technologies, making it ideal for use not only in the United States but in developing countries, which often face water quality issues.
At home and abroad, animal and human waste can contaminate both recreational and source waters, carrying with diseases such as polio, typhoid and cholera. This form of contamination can even result in environmental crises, such as devastation to the aquatic population and red-tide blooms. Yakovlev notes. These types of contamination events, Yakovlev explains, might be mitigated or even avoided if samples from water systems are more thoroughly analyzed so that they can provide a better picture of what is in the water. In other words, finding trace amounts of contaminants such as fecal matter in water systems can help sound the alarm for a serious contamination event because these trace amounts likely originate from a larger source in the water system, he notes.
However, detecting these trace amounts isn’t easy, especially in a timely manner, Yakovlev says. High costs, sample-size limitations and lengthy analysis times, he notes, have prevented environmental researchers from employing highly sensitive techniques that can deliver real-time analysis – until now.
Yakovlev and his colleagues are poised to change things with an innovative approach to detecting something known as urobilin. Urobilin is a byproduct excreted in the urine and feces of many mammals, including humans and livestock such as cows, horses and pigs. Urobilin molecules, Yakovlev notes, are small and diffuse quickly so they easily occupy large volumes, such as lakes and reservoirs, for example.
In addition, urobilin possesses another interesting property; it glows – or more accurately, it can be made to glow. When mixed with zinc ions, urobilin forms a phosphorescent compound, Yakovlev explains. This means if urobilin is present in a water sample – and zinc ions have been added – the sample will give off a greenish glow when examined under an ultraviolet light, he says. There’s just one catch. In some samples with low concentrations of urobilin, the glow, or phosphorescent emission, can be weak, making it difficult to analyze the sample. Researchers, Yakovlev says, must be able to thoroughly excite the sample (causing the reaction), observe the glow and then measure it in order to perform an accurate analysis.
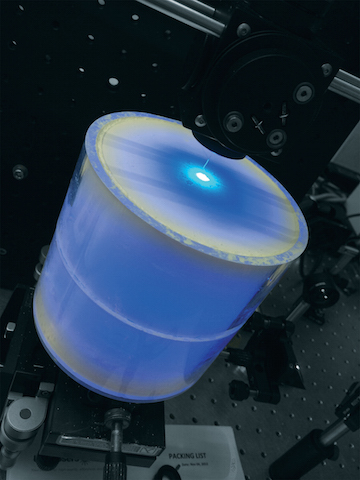
Towards that goal, Yakovlev and his team have developed technology that allows them to thoroughly excite extremely small amounts of urobilin in large samples of water and then efficiently collect the resulting phosphorescent emission, regardless of how weak that emission might be. It’s done with the help of device researchers refer to as an “integrated cavity.”
The integrated cavity used by the team of researchers is essentially a hollow, cylindrical container manufactured in Yakovlev’s laboratory. A water sample is placed inside the cylinder where it interacts with zinc ions, and a laser light is beamed into the object and onto the sample through a small hole, Yakovlev explains. The light excites the urobilin compound present in the sample, causing it to emit a glow. The only way for the light to exit the cylinder, he notes, is through the hole that it initially entered. Not only does this ensure that all the light that enters the cylinder is used to excite the entire sample, it also enables researchers to efficiently collect the resulting phosphorescent emission so that it can be directed to a photo detector, such as a spectrometer, for analysis, Yakovlev says.
Employing the integrated cavity in their detection efforts, Yakovlev and his team have detected the presence of urobilin down to a nanomole per liter. A mole is a common unit of measurement in chemistry, and a nanomole is one billionth of that measurement. What’s more the technology provides actual concentration levels of the contaminant, and it does so much quicker than other methods, he notes.
“We can demonstrate detection of ultralow concentrations of urobilin in solution,” Yakovlev says. “This is a huge improvement in terms of sensitivity, and our technique has tremendous potential for analysis of global drinking water supplies, particularly in developing nations and following natural disasters, where sophisticated laboratory equipment may not be available.”
Another key element of the technology, which can be produced for a few hundred dollars, is its ability to analyze large samples, Yakovlev notes. Conventional methods are not capable of analyzing large samples. This is a problem, he adds, because it is unlikely that an accurate analysis of an overall water system can be derived from a small sample. For example, researchers might collect a small sample that is free of the contaminant, but that doesn’t mean the entire water system is contaminant free. A larger sample, he says, gives researchers a better predictive power about the water system contains.
As its stands, Yakovlev and his team are working to commercialize the technology for urobilin detection. Because it delivers nearly instantaneous results, it could serve as the basis for in-home detection systems that alert users if the water coming from their faucets is suddenly contaminated, he says. Think smoke detector for a water faucet. Equally as important, he notes, the technology can be used for detection of other types of toxic compounds in both liquids and gases, lending itself to anti-terrorism applications, among other uses.
Yakovlev’s collaborators are Marlan O. Scully and Edward S. Fry, distinguished professors in the Department of Physics and Astronomy at Texas A&M; and graduate students Joel N. Bixler, Michael Cone, Brett Hokr, John Mason and Ellie Figueroa.
Contact: Professor Vladislav Yakovlev at 979.458.2326 or via email: [email protected].